Procaine Hydrochloride (HCl) Injection:
This document is intended for informational purposes only and does not provide
medical advice, treatment recommendations or therapeutic claims.
1. INTRODUCTION
Procaine Hydrochloride (Procaine HCl) is an ester-type local anesthetic first introduced in the early 1900s and extensively described in pharmacologic literature for its ability to produce reversible blockade of peripheral nerve conduction,2,3
The compound consists of an amino ester linked to para-aminobenzoic acid (PABA), giving it physicochemical and metabolic properties distinct from amide anesthetics such as lidocaine or bupivacaine.2,3
Because commercial sterile formulations may not always be available, healthcare institutions may utilize compounded sterile preparations when appropriate and compliant with regulatory standards. This educational review summarizes Procaine’s chemistry, pharmacology, physicochemical behavior, stability profile, and safety considerations, supported by primary literature, pharmacopeial monographs, and recognized anesthesia pharmacology references.
2. CHEMISTRY AND PHYSIOLOGICAL ROLE
Chemical Identity
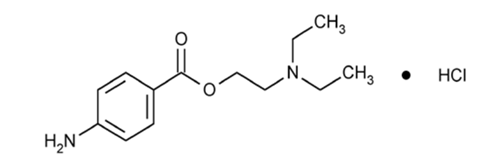
Procaine Hydrochloride (C₁₃H₂₀N₂O₂·HCl) is a synthetic amino-ester local anesthetic presented as a white to off-white, crystalline, water-soluble hydrochloride salt with characterization parameters established in the United States Pharmacopeia (USP).1 Key structural components include:
-
Para-aminobenzoic acid (PABA) aromatic ring
-
Benzoic acid ester linkage
-
Tertiary diethylaminoethanol group
This arrangement determines its classification as an ester-type anesthetic and drives its characteristic ionization and metabolic pathways2,3
Ionization Behavior
Procaine has a pKa of approximately 8.9, meaning that at physiologic pH (6.35–7.45), it exists predominantly in its protonated (ionized) form.3 This influences:
-
Membrane permeability
-
Onset of action
-
Duration of effect
-
Binding affinity to voltage-gated sodium channels
Chemical Instability of Ester Linkage
The ester bond is the principal site of chemical degradation and enzymatic hydrolysis. Hydrolysis susceptibility of ester compounds is influenced by pH, temperature, and time in aqueous solution, consistent with general ester-chemistry principles rather than Procaine-specific validated stability data.2,3
Degradation yields PABA and diethylaminoethanol.2,6 This hydrolysis reaction also occurs in vivo through rapid metabolism by plasma pseudocholinesterase, contributing to Procaine’s short systemic persistence and limiting plasma accumulation3,6
Physiologic Interaction
Procaine is not endogenous and has no natural physiologic role but interacts with sodium-channel physiology when administered. The ionized drug binds voltage-gated sodium channels, inhibits depolarization, and temporarily blocks nerve conduction.2,3
PABA, a metabolic product, is associated with hypersensitivity reactions and may antagonize sulfonamide antibiotics.6,7
3. PHARMACOLOGY AND MECHANISM OF ACTION
Procaine HCl acts by reversibly blocking voltage-gated sodium channels, increasing the threshold for neuronal depolarization and slowing propagation of action potentials.2,4
Binding occurs at the intracellular side of the sodium channel, stabilizing the inactivated state and limiting sodium influx.3,4
This process is:
-
Frequency-dependent
-
Concentration-dependent
-
Influenced by drug ionization and local tissue pH
These mechanistic descriptions reflect pharmacologic principles and should not be interpreted as clinical performance guarantees or dosing guidance.
4. PHARMACOKINETICS
Absorption
Systemic absorption varies with dose, vascularity, and injection site.
More vascular tissues demonstrate higher systemic uptake.2
Distribution
Like other ester anesthetics, Procaine exhibits rapid systemic distribution after absorption.
Low plasma protein binding contributes to its short duration of systemic exposure.3
Metabolism
Procaine is rapidly hydrolyzed by plasma pseudocholinesterase into PABA and diethylaminoethanol.
Individuals with genetic or acquired pseudocholinesterase deficiency may experience prolonged effects.3,4
Elimination
Metabolites are excreted predominantly in urine.7
5. PHYSICOCHEMICAL PROPERTIES AND STABILITY
Chemical Stability and Hydrolysis
Procaine Hydrochloride contains an ester functional group that is chemically susceptible
to hydrolysis, consistent with the broader class of ester-type local anesthetics.2,3
Hydrolysis results in the formation of para-aminobenzoic acid (PABA) and
diethylaminoethanol (DEAE).2,3
Hydrolysis Pathway (Class-Based Behavior)
The ester linkage may undergo hydrolytic degradation influenced by factors known to affect
ester stability, including pH, temperature, aqueous environment, and the duration of time
in solution.2,3
Alkaline pH conditions are associated with increased ester cleavage, while comparatively
acidic environments are more favorable for stability.2,3
These characteristics reflect general ester chemistry principles and may not represent
Procaine-specific validated kinetic data.
Light and Visual Appearance Considerations
Published Procaine-specific data regarding light sensitivity are limited. Nevertheless,
minimizing unnecessary light exposure is a commonly applied precaution in pharmaceutical
handling of ester-containing solutions. Solutions are generally expected to appear clear
and colorless; visible discoloration or particulate matter warrants investigation or
disposal in accordance with applicable USP and institutional quality procedures.1
Limitations of Stability Principles
These principles describe expected behavior of ester-containing molecules and are not a
substitute for formulation-specific stability studies, beyond-use dating, or
container-closure validation.
6. HISTORICAL & INVESTIVATIONAL USES
Procaine Hydrochloride has long been used as a short-acting ester local anesthetic.
Beyond its established anesthetic role, Procaine has appeared in exploratory and
complementary medicine literature, particularly in neural therapy, integrative
multimodal injection-based practices, and anti-aging hypotheses.
The following subsections summarize investigational areas in which Procaine has been
evaluated. These descriptions reflect published inquiry only and do not indicate
therapeutic endorsement or regulatory approval.
6.1 Neural Therapy (Local Procaine Injections)
Neural therapy involves injecting dilute Procaine into scars, dermatomes, trigger
points, or autonomic regions with the goal of modulating nociceptive or autonomic
dysfunction. Several clinical investigations have evaluated this approach.
An observational pain-center cohort (n=280) reported improvement in chronic pain
symptoms following neural-therapy injections using local anesthetics, including
Procaine.10 Because the study lacked a control group, results cannot
determine causality.
A randomized controlled trial evaluating 1% Procaine injections for supraspinatus
tendinopathy demonstrated short-term reductions in pain and improved function from
baseline.11
Evidence reviews have concluded that although neural therapy is practiced
internationally, the available clinical evidence is heterogeneous and underpowered,
resulting in insufficient high-quality evidence to determine efficacy.12
Overall, evidence suggests possible short-term symptomatic benefit in select
musculoskeletal conditions, but findings remain preliminary and inconsistent.
6.2 Regenerative and Prolotherapy-Associated Injection Procedures
Procaine is occasionally used as an anesthetic component within multimodal
injection-based pain treatments in integrative medicine. Reviews of injection-based
therapies describe short-acting local anesthetics, including Procaine, as adjunct
agents used for procedural analgesia, modulation of nociceptive input, or facilitation
of needling techniques.9
In regenerative procedures such as prolotherapy, local anesthetics may be incorporated
for patient comfort or procedural support; however, existing literature does not
identify Procaine as a primary therapeutic component of prolotherapy. Its involvement
in these modalities is considered adjunctive and procedural rather than regenerative,
consistent with general anesthetic use rather than Procaine-specific therapeutic
effects.9
6.3 Anti-Aging and “Geroprotector” Hypotheses
Procaine has historically been associated with anti-aging claims, partly due to
formulations such as “Gerovital H3.” Modern scientific assessments have
reevaluated these claims using contemporary standards.
A 2021 critical review characterized Procaine as a “controversial geroprotector
candidate,” citing inconsistent and nonreproducible findings across earlier studies,
significant methodological limitations in historical literature, and a lack of
evidence supporting systemic anti-aging or longevity effects.13
The investigational and historical uses described in this section reflect published
literature only. They do not constitute medical advice, do not imply proven clinical
benefit, and are not recognized indications.
7. SAFETY PROFILE & ADVERSE EFFECTS
Expected Class-Related Adverse Effects
These reactions are described in anesthesiology literature and represent expected
pharmacologic responses associated with sodium-channel blockade.2–3,6
Reported effects include local injection-site discomfort or burning, mild erythema
or swelling, tingling or altered sensation, and temporary localized numbness.
These effects are typically self-limited and related to route of administration
and injection technique.
Hypersensitivity
Metabolism of Procaine produces para-aminobenzoic acid (PABA), and ester anesthetics
are therefore associated with a higher likelihood of hypersensitivity reactions.2,3
Reported reactions include rash, urticaria, pruritus, bronchospasm, and rare severe
allergic reactions. This represents a known class effect among ester anesthetics.
Dose-Related Systemic Toxicity
Systemic toxicity is dose-dependent, correlates with plasma concentrations, and
commonly involves the central nervous system and cardiovascular system.2–4
Central nervous system effects may include tinnitus, dizziness, tremors, circumoral
numbness, and seizures.
Cardiovascular system effects may include hypotension, bradycardia, conduction
abnormalities, and rare cardiovascular collapse. These effects are class-related
and described for all local anesthetics.
Injection- or Infusion-Related Reactions
Reported reactions associated with local infiltration or vascular administration
include local tissue irritation, hematoma formation, and phlebitis or venous
irritation during intravenous infusion.1,3
These reactions are not unique to Procaine.
Drug Interactions
Para-aminobenzoic acid (PABA) may antagonize sulfonamide-class antibiotics, representing
a recognized biochemical interaction.3,6
Special Populations – Pseudocholinesterase Deficiency
Individuals with congenital or acquired pseudocholinesterase deficiencies may
experience prolonged anesthetic duration due to impaired ester metabolism.2–4
8. FORMULATION & HANDLING CONSIDERATIONS
Procaine, similar to other ester-type anesthetics, is subject to hydrolytic degradation
in aqueous environments, and this process may be influenced by pH, temperature, and
duration in solution. Stability is generally greater in acidic conditions, whereas
alkaline environments may accelerate ester cleavage.2,3
Although Procaine-specific light- or thermal-degradation studies are limited,
minimizing unnecessary exposure to direct light and heat is consistent with general
chemical handling practices for ester-containing solutions.
Procaine solutions should remain clear and colorless; any discoloration or visible
particulate matter warrants investigation or disposal in accordance with USP
standards and facility quality procedures.1
These considerations are scientific in nature and are not preparation, storage, or
beyond-use-date instructions. Actual storage conditions, beyond-use dating, and
container-closure requirements must be based on manufacturer labeling, applicable
regulations, and validated stability programs.
9. SUMMARY
Procaine Hydrochloride (Procaine HCl) is an ester-type local anesthetic with
well-described chemical and pharmacologic properties that are associated with
characteristically rapid onset, short duration of systemic exposure, and rapid
metabolic clearance.2–3,5
Its mechanism of action involves reversible blockade of voltage-gated sodium channels
with preferential affinity for the inactivated state, resulting in temporary
interruption of neuronal signal conduction and nociceptive transmission.2–4
Although historical literature describes use in various anesthetic, analgesic, and
neuromodulatory contexts,2–5 contemporary evidence remains limited, and
much of the published work reflects historic practice patterns, exploratory and
mechanistic research, or niche applications outside mainstream clinical
protocols.5–6,9
From a clinical safety perspective, Procaine shares class-based adverse event risks
associated with local anesthetics, including potential hypersensitivity reactions
due to para-aminobenzoic acid (PABA) metabolite formation,3,5–6 and rare but
serious systemic toxicity when excessive plasma concentrations occur.3,5–7
Use outside standard anesthetic practice settings—such as investigational, cosmetic,
alternative-medicine, or non-regulated wellness applications—underscores the
importance of validated formulation quality, professional oversight, controlled
administration settings, and adherence to applicable regulatory standards and
compounding requirements.1,5,7,9
Further well-designed, controlled studies would be valuable to clarify Procaine’s
contemporary therapeutic roles, safety parameters, and comparative utility relative
to currently utilized anesthetic agents, as well as to evaluate emerging mechanistic
hypotheses under rigorously monitored research conditions.5,9
10. REFERENCES
-
United States Pharmacopeia (USP). Procaine Hydrochloride Monograph.
Rockville, MD: United States Pharmacopeial Convention; 2022.
-
Covino BG, Vassallo HG. Local Anesthetics: Mechanisms of Action and Clinical Use.
New York, NY: Grune & Stratton; 1976.
-
Becker DE, Reed KL. Essentials of local anesthetic pharmacology.
Anesth Prog. 2006;53(3):98–109.
-
Butterworth JF IV, Strichartz GR. Molecular mechanisms of local anesthesia.
Anesthesiology. 1990;72(4):711–734.
-
Sheikh NK, Dua A. Procaine. In: StatPearls [Internet].
Treasure Island (FL): StatPearls Publishing; 2025 Jan–.
Updated May 8, 2023.
-
Yagiela JA. Local anesthetics: a century of progress.
Anesth Prog. 1985;32(2):46–56.
-
El-Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity:
current perspectives. Local Reg Anesth. 2018;11:35–44.
-
Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations.
Anesth Prog. 2012;59(2):90–102.
-
Vinyes D, Muñoz-Sellart M, Fischer L. Therapeutic use of low-dose local anesthetics
in pain, inflammation, and other clinical conditions: a systematic scoping review.
J Clin Med. 2023;12(23):7221.
-
Egli S, Pfister M, Ludin SM, et al. Long-term results of therapeutic local anesthesia
(neural therapy) in 280 referred refractory chronic pain patients.
BMC Complement Altern Med. 2015;15:200.
-
Bashan I, Ozturk GY. Effect of neural therapy on shoulder dysfunction and pain in
supraspinatus tendinopathy. Pak J Med Sci. 2022;38(3 Pt I):565–569.
-
Weinschenk S. Neural therapy: a review of the therapeutic use of local anesthetics.
Acupunct Relat Ther. 2012;1(1):5–9.
-
Gradinaru D, Ungurianu A, Margina D, Moreno-Villanueva M, Bürkle A.
Procaine—the controversial geroprotector candidate: new insights regarding its
molecular and cellular effects. Oxid Med Cell Longev. 2021;2021:3617042.
Copyright © 2025 McGuff Outsourcing Solutions. All rights reserved.
No part of this document may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or electronic transmission, without the prior written permission of McGuff Outsourcing Solutions.