Methylcobalamin:
This document is intended for informational purposes only and does not provide
medical advice, treatment recommendations or therapeutic claims.
1. INTRODUCTION
Vitamin B12 is an essential nutrient involved in normal neurologic function, red blood cell formation, and a variety of metabolic processes. Among its forms, methylcobalamin (MeCbl) is one of the biologically active coenzymes used in the body’s methylation pathways, including the conversion of homocysteine to methionine.
Because vitamin B12 status can be influenced by age, diet, gastrointestinal absorption, and certain medications, reductions in circulating levels may lead to elevations in homocysteine and methylmalonic acid—biochemical markers commonly used to assess B12 adequacy.1
Scientific publications describe MeCbl as the cobalamin form active within the cytosol, and experimental studies have explored its cellular handling, transport, and behavior in laboratory models. Early clinical and mechanistic research has examined MeCbl in contexts involving nerve conduction, myelin support, and cellular responses to metabolic stress2
Parenteral administration of vitamin B12 is discussed in the literature as a method of delivering cobalamin without reliance on intrinsic factor–dependent absorption. Published work has evaluated how injected cobalamin is taken up, distributed, and metabolized across different tissues and physiological pathways.1,2
This document provides an educational overview of the pharmacology, physiological role, reported clinical investigations, and safety considerations related to MeCbl. Discussion of published studies is descriptive only and does not imply established clinical benefit or regulatory approval for any specific indication.
2. CHEMISTRY AND PHYSIOLOGICAL ROLE
MeCbl is characterized by a corrin-ring cobalt center bound to a methyl ligand (Figure 1), enabling the formation and cleavage of a cobalt–carbon bond critical for methyl group transfer. Physiologically, MeCbl serves as the required cofactor for methionine synthase, the enzyme that catalyzes the remethylation of homocysteine to methionine and supports the generation of S-adenosylmethionine (SAM), the universal methyl donor for DNA and protein methylation. Through its role in the one-carbon cycle, MeCbl is essential for maintaining DNA methylation patterns, regulating gene expression, and supporting nucleotide synthesis. Deficiency impairs these pathways, leading to SAM depletion, genomic hypomethylation, uracil misincorporation, and can contribute to genomic instability.
In addition, cobalamins—including MeCbl—function as intracellular antioxidants by scavenging reactive oxygen species and preserving glutathione stores, thereby reducing oxidative DNA injury and contributing to overall genome stability.3
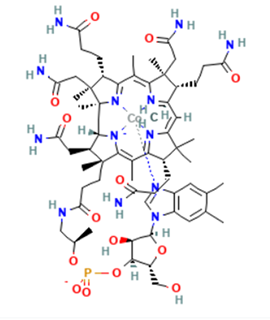
Figure 1. Chemical structure of MeCbl
3. PHARMACOLOGY AND MECHANISM OF ACTION
MeCbl is one of the two active coenzyme forms of vitamin B12 and serves as the required cofactor for methionine synthase (MS), the enzyme that remethylates homocysteine to methionine.
Through this pathway, MeCbl contributes to folate metabolism, S-adenosylmethionine (SAM) generation, and a broad range of methylation reactions involving DNA, proteins, lipids, and myelin components.3
When MeCbl-dependent MS activity is reduced—whether through dietary insufficiency, malabsorption, or impaired cobalamin metabolism—biochemical consequences described in the literature include elevated homocysteine, decreased SAM, altered methylation patterns, increased uracil misincorporation, and can contribute to genomic instability.1,3
These disruptions are associated with several physiologic and neurologic findings reported in B12 insufficiency, such as myelin degeneration, axonal injury, abnormal nerve conduction, and the characteristic neuropathological features of subacute combined degeneration.1,4
Laboratory and experimental publications also describe neuronal uptake of MeCbl and report measurements involving axonal structures, myelin-supporting processes, and cellular responses to metabolic and excitatory stress, including glutamate exposure in vitro and in vivo.2
These investigations reflect the broader biologic context in which cobalamin-dependent methylation supports normal neurologic and hematologic function.
4. PHARMACOKINETICS MeCbl
MeCbl follows established cobalamin pharmacokinetics. Parenteral administration bypasses intrinsic-factor–dependent absorption, providing delivery that is unaffected by malabsorption or impaired gastric function.4
After entering circulation, MeCbl binds transcobalamin II (TCII) to form holotranscobalamin (holoTC), enabling cellular uptake through CD320 receptor–mediated endocytosis, followed by lysosomal processing and intracellular trafficking to B12-dependent enzymes.1
Vitamin B12 is widely distributed in tissues and stored predominantly in the liver, contributing to its slow turnover and long biological retention time.4
5. HISTORICAL AND INVESTIGATIONAL CLINICAL USES OF MeCbl
MeCbl has been used in clinical studies for several decades as one of the parenteral forms of vitamin B12 and has been investigated in neurologic and pain-related contexts beyond classical B12 deficiency.
Historical and contemporary trials have evaluated intramuscular, intravenous, and local subcutaneous administration of MeCbl in conditions such as amyotrophic lateral sclerosis, peripheral neuropathy, neuropathic pain, and autism spectrum disorder.2,6
These uses are reported as investigational, and the available studies are generally limited by small sample sizes, relatively short treatment durations, and, in several cases, open-label or otherwise nonblinded designs. Inclusion of a condition indicates published investigation, not demonstrated therapeutic benefit.
5.1 Amyotrophic lateral sclerosis (ALS)
Ultrahigh-dose intramuscular MeCbl has been evaluated in clinical research as a potential adjunctive approach in early amyotrophic lateral sclerosis (ALS).
In the Japan Early-Stage Trial of Ultrahigh-Dose MeCbl (JETALS), patients within one year of symptom onset were randomized to receive treatment drug or placebo in a double-blind, placebo-controlled phase 3 design.
Over the 16-week treatment period, the decline in ALS Functional Rating Scale–Revised (ALSFRS-R) scores was smaller in the MeCbl group than in the placebo group, and adverse event rates were similar between groups.6
5.2 Peripheral neuropathy and axonal degeneration
A phase I/II open-label trial evaluated 14 patients with chronic immune-mediated or hereditary neuropathies and electrodiagnostic evidence of axonal degeneration. The primary objectives were to assess safety and explore changes in motor function using the Medical Research Council (MRC) sum score. Intravenous ultra–high-dose MeCbl produced markedly elevated serum vitamin B12 concentrations and was generally well tolerated. Among the 12 evaluable patients, 7 showed increases in MRC sum scores, with some of the larger changes noted among immune-mediated neuropathies.
However, the lack of a control group, open-label design, and small sample size limit interpretation of these findings, and intravenous MeCbl for this indication remains experimental.7
5.3 Neuropathic and musculoskeletal pain
Methylcobalamin has been explored in several clinical studies as a potential adjunct in conditions involving neuropathic or musculoskeletal pain.
In a randomized, double-blind trial of chronic nonspecific low back pain, intramuscular MeCbl was associated with greater improvements in disability scores and visual analog pain ratings compared with placebo, with only minor injection-site reactions reported.11
In subacute herpetic neuralgia, a single-center randomized study compared local subcutaneous MeCbl with oral MeCbl and with local lidocaine.
The locally administered MeCbl group reported larger reductions in multiple pain measures—including continuous pain, paroxysmal pain, allodynia, and analgesic use—and also showed improvements in functional assessments.
Oral MeCbl did not differ significantly from control for pain outcomes in this study.10
A broader review of the literature describes additional small or heterogeneous studies evaluating MeCbl in diabetic neuropathy, neuralgia, lumbar spinal stenosis–related symptoms, and other pain conditions.
These reports note that evidence remains limited, variable, and largely exploratory.2
5.4 Autism spectrum disorder (ASD)
A randomized, placebo-controlled study in children with autism spectrum disorder (ASD) evaluated subcutaneous MeCbl administered every three days for eight weeks.
Clinician-rated improvement on the Clinical Global Impressions–Improvement (CGI-I) scale favored MeCbl and was accompanied by changes in biomarkers related to methylation capacity, such as the SAM:SAH ratio.
In contrast, parent-reported behavioral measures, including the Aberrant Behavior Checklist and Social Responsiveness Scale, did not show significant differences between groups.
The trial was relatively small, short in duration, and limited to children with IQ > 50; therefore, MeCbl remains an investigational, mechanism-focused approach in ASD rather than an established treatment.8
Overall, intramuscular and subcutaneous MeCbl is generally well tolerated. However, most studies are small, of limited duration, and not powered to establish definitive efficacy.
6. SAFETY PROFILE AND ADVERSE EFFECTS
Across published parenteral MeCbl studies in adolescents and adults, treatment has generally been described as well tolerated, with most reported adverse events typically mild and transient. Observed reactions include:
Injection-Site Reactions
-
Pain at injection site10,11
-
Bruising/Contusion6
-
Hematoma 11
Skin/Allergic
-
Rash8
-
Hypersensitivity reaction12
-
Dermatitis7
Systemic
-
Urine discoloration (red)99
Across the clinical studies reviewed, no drug-related serious adverse reactions were identified, and only mild and transient adverse events were observed.
7. FORMULATION, STABILITY, AND HANDLING (SCIENTIFIC OVERVIEW)
MeCbl is a light-sensitive and oxidation-labile form of vitamin B12 that can undergo degradation in aqueous solution through photolysis and oxidative pathways.
Because parenteral MeCbl preparations must also meet stringent sterility and endotoxin limits, these characteristics have direct implications for formulation development, compounding, and storage:
Light Sensitivity
MeCbl readily undergoes photodegradation when exposed to ambient or ultraviolet light, leading to loss of potency and formation of breakdown products. MeCbl can convert to hydroxocobalamin when exposed to light.
As a result, formulation strategies generally include protection from light during preparation (e.g., amber light environments), the use of amber-colored containers, and secondary light-protective packaging during storage and transport.
Oxidation
As a reduced cobalamin species, MeCbl can oxidize to hydroxocobalamin and other cobalamin derivatives in the presence of oxygen, trace metal ions, or other catalytic impurities.
To minimize oxidative degradation, aqueous preparations may be compounded under inert or low-oxygen conditions, with careful control of dissolved oxygen and consideration of compatible excipients that do not catalyze cobalamin oxidation.
Sterility and Endotoxin Control
MeCbl injectable products are sterilized by aseptic filtration rather than terminal sterilization.
Consequently, validated aseptic processing controls—validated sterile filtration, environmental monitoring, container–closure integrity assurance, and strict microbial and endotoxin specifications—are critical to ensuring the safety and quality of parenteral-grade solutions.
8. REGULATORY AND QUALITY CONSIDERATIONS (HIGH LEVEL SUMMARY)
Regulatory agencies in the United States have issued communications and observations related to the compounding and clinical use of MeCbl for injection. Any sterile MeCbl injection available in the United States must be compounded, as no FDA-approved MeCbl injectable product exists. Regulatory discussions commonly emphasize:
Raw Material Quality
Authorities have highlighted risks associated with the use of non–pharmaceutical-grade MeCbl or bulk dietary-supplement ingredients as starting materials for injectable formulations.
Issues cited include potential microbial contamination, elevated endotoxin levels, variable potency, and insufficient control of impurities—all of which are inconsistent with requirements for parenteral-grade active substances.
Compounded injectable preparations should therefore use only pharmaceutical-grade API that complies with applicable compendial and regulatory specifications.
Compliance with Sterile Manufacturing Standards
Because MeCbl injections are administered parenterally, regulatory expectations require adherence to stringent sterile manufacturing standards, including current Good Manufacturing Practice (GMP) or equivalent standards for 503B outsourcing facilities. These expectations include:
-
Validated aseptic processing and/or sterile filtration
-
Appropriate environmental monitoring and aseptic-process simulation (media fills)
-
Control of light exposure, given the compound’s photolability
-
Container–closure integrity verification
-
Comprehensive quality control testing, including sterility, endotoxin, particulate matter, pH, and potency
Regulators have emphasized that failure to meet these sterile-manufacturing requirements for compounded MeCbl may pose risks to patient safety.
Practitioner Awareness and Regulatory Compliance
Healthcare practitioners should be aware of relevant federal, state, and local regulations governing the sourcing, compounding, prescribing, and distribution of MeCbl for injection, including requirements that apply specifically to 503B outsourcing facilities and 503A compounding pharmacies.
MeCbl injectable preparations should be used only when they are sourced from facilities operating under compliant sterile-processing and quality-assurance systems, and when the product’s formulation and handling practices align with established regulatory expectations.
9. SUMMARY
MeCbl is an active form of vitamin B12 essential for DNA methylation, neurologic function, and prevention of homocysteine-related toxicity.1-5
Its preferential neuronal uptake and role in one-carbon metabolism support myelin integrity and genomic stability, with deficiency associated with neurologic dysfunction, including neuropathy, and oxidative stress.1-4
Parenteral MeCbl bypasses absorption barriers and has been investigated for neuropathy, neuralgia, ALS, autism, and pain, though evidence across these indications remains preliminary and investigational2,6-9
Across the reviewed studies, MeCbl was generally well tolerated, with no drug-related serious adverse reactions identified.6-12
Because the compound is light- and oxidation-sensitive, injectable preparations require protective handling, aseptic sterile filtration, and compliance with regulatory standards for raw-material quality and sterile manufacturing.
10. SELECTED REFERENCES
-
Umekar M, Premchandani T, Tatode A, Qutub M, Raut N, Taksande J, Hussain UM. Vitamin B12 deficiency and cognitive impairment: A comprehensive review of neurological impact. Brain Disord. 2025;18:100220. doi:10.1016/j.dscb.2025.100220.
-
Zhang M, Han W, Hu S, Xu H. Methylcobalamin: A potential vitamin of pain killer. Neural Plast. 2013;2013:1-6. doi:10.1155/2013/424651.
-
Hałczuk I, Sokołowska E, Strzadała L. Vitamin B12—Multifaceted in vivo functions and in vitro applications. Nutrients. 2023;15(16):3534. doi:10.3390/nu15163534.
-
Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149-160. doi:10.1056/NEJMcp1113996.
-
Banerjee R, Ragsdale SW. The many faces of vitamin B12: Catalysis by cobalamin-dependent enzymes. Annu Rev Biochem. 2003;72(1):209-247. doi:10.1146/annurev.biochem.72.121801.161828.
-
Oki Y, Hashizume A, Hijikata Y, et al. Ultra-high dose methylcobalamin in amyotrophic lateral sclerosis: A randomized controlled trial. JAMA Neurol. 2022;79(6):575-583. doi:10.1001/jamaneurol.2022.0832.
-
Shibuya K, Misawa S, Arai K, et al. Safety and efficacy of intravenous ultra-high-dose methylcobalamin treatment for peripheral neuropathy: A phase I/II open-label clinical trial. Intern Med. 2014;53(17):1927-1931. doi:10.2169/internalmedicine.53.2607.
-
Hendren RL, James SJ, Widjaja F, et al. Randomized, placebo-controlled trial of methyl B12 in children with autism. J Child Adolesc Psychopharmacol. 2016;26(8):774-783. doi:10.1089/cap.2015.0156.
-
Sall Hansson K, Bådagård H, Elvin K, et al. Efficacy of mecobalamin (vitamin B12) in the treatment of long-term pain in women diagnosed with fibromyalgia: Protocol for a randomized, placebo-controlled trial. BMJ Open. 2023;13(5):e066987. doi:10.1136/bmjopen-2022-066987.
-
Xu J, Chen S, Zhao H, Liang J, Wang Y. A single-center randomized controlled trial of local methylcobalamin injection for subacute herpetic neuralgia. Pain Med. 2013;14(5):884-894. doi:10.1111/pme.12024.
-
Chiu CK, Low TH, Tey YS, et al. The efficacy and safety of intramuscular injections of methylcobalamin in patients with chronic nonspecific low back pain: A randomized controlled trial. Singapore Med J. 2011;52(12):868-873.
-
Gupta JK, Qureshi R. Potential benefits of methylcobalamin: A review. Austin J Pharmacol Ther. 2015;3(1):1065
Copyright © 2025 McGuff Outsourcing Solutions. All rights reserved.
No part of this document may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or electronic transmission, without the prior written permission of McGuff Outsourcing Solutions.