Intravenous Reduced L-Glutathione (GSH)
This document is intended for informational purposes only and does not provide
medical advice, treatment recommendations or therapeutic claims.
1. INTRODUCTION
Glutathione (GSH) is a tripeptide composed of glutamate, cysteine, and glycine, and is the most abundant low-molecular-weight thiol in human cells. It plays a central role in maintaining cellular redox balance, detoxifying reactive intermediates, and supporting normal immune and metabolic function.
1-3
Disturbances in glutathione homeostasis have been associated with oxidative stress and a wide range of pathophysiologic conditions.1-2 Because oral glutathione exhibits limited systemic bioavailability,4 intravenous (IV) glutathione has been investigated in several small clinical and exploratory research settings.
This document summarizes the pharmacology, pharmacokinetics, reported investigational uses, and safety considerations associated with IV glutathione, drawing from the available scientific and clinical literature.
The focus of this review is educational. Discussion of clinical studies is descriptive only and should not be interpreted as implying established efficacy, regulatory approval, or specific treatment recommendations for any indication.
2. CHEMISTRY AND PHYSIOLOGICAL ROLE
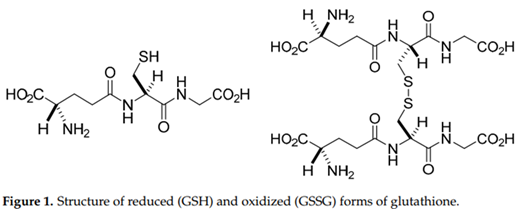
Glutathione exists primarily in its reduced form (GSH) under normal physiological conditions, with a smaller fraction present as the oxidized disulfide form (GSSG).1-3 Figure 1 shows the molecular structure of GSH and GSSG.
The intracellular GSH:GSSG ratio is often used as a marker of cellular redox status1. GSH participates in enzymatic reactions involving glutathione peroxidases, glutathione S-transferases, and glutathione reductase, among others.5
GSH is synthesized endogenously in a two-step ATP-dependent pathway from its constituent amino acids.3 It is highly concentrated in the liver but is also present in significant amounts in the lungs, kidneys, gastrointestinal tract, and central nervous system.
GSH is involved in detoxification of xenobiotics, conjugation of reactive metabolites, maintenance of thiol status of proteins, and modulation of inflammatory and immune pathways.2
Disturbances in GSH homeostasis have been associated with conditions in which oxidative stress, inflammation, or metabolic dysfunction play a role, including neurodegenerative diseases, chronic respiratory disorders, cardiovascular disease, and metabolic syndrome.1-3
These observations have led to scientific interest in whether exogenous glutathione administration can influence redox status.
3. PHARMACOLOGY AND MECHANISM OF ACTION
The pharmacological effects of exogenous glutathione largely derive from its capacity to participate in redox reactions and conjugation pathways. Key mechanisms described in the literature include:
-
Antioxidant activity: GSH donates electrons to reactive oxygen and nitrogen species, limiting oxidative damage to lipids, proteins, and nucleic acids.
In doing so, it is converted to GSSG, which can be reduced back to GSH by glutathione reductase using NADPH.2,3,5
-
Detoxification: Through glutathione S-transferases, GSH conjugates electrophilic metabolites and xenobiotics, facilitating their excretion via bile or urine.3,5
-
Redox signaling and enzyme regulation: GSH helps maintain the redox state of cysteine residues within proteins, influencing the activity of various enzymes and transcription factors involved in inflammation, apoptosis, and cell stress response.2,5
-
Support of other antioxidant systems: Adequate GSH stores are required to maintain the function of other antioxidant defenses, including the detoxification of hydrogen peroxide via glutathione peroxidase.5
Because endogenous glutathione levels are tightly regulated, intravenous administration leads to a transient rise in circulating GSH that may influence these biochemical pathways for a limited period following infusion.
4. PHARMACOKINETICS OF INTRAVENOUS GLUTATHIONE
Pharmacokinetic studies in humans indicate that intravenous glutathione is rapidly distributed and cleared from the circulation. In evaluations involving both healthy volunteers and patients receiving high-dose IV GSH, plasma glutathione concentrations increased sharply during infusion and declined quickly once administration ceased, with a reported plasma half-life on the order of minutes to tens of minutes.6
These studies also demonstrated rapid oxidation of reduced GSH to GSSG and other downstream metabolites.
Urinary excretion of glutathione-related metabolites, including GSH and cysteine, increases significantly after high-dose IV administration, reflecting renal handling and elimination of these compounds.6
Because endogenous glutathione levels are tightly regulated, IV administration produces only a transient rise in circulating GSH. As a result, IV glutathione is typically administered intermittently in investigational clinical settings rather than as a continuous infusion.
The short duration of plasma elevation is an important consideration when interpreting the time course of any pharmacodynamic effects reported in clinical studies.
5. HISTORICAL AND INVESTIGATIONAL CLINICAL USES OF IV GLUTATHIONE
Intravenous glutathione has been investigated across a variety of conditions where oxidative stress and inflammation are believed to contribute to pathophysiology.
The following subsections summarize selected areas of study. Inclusion of a condition indicates published investigation, not established therapeutic benefit.
5.1 Neurodegenerative Disorders (e.g., Parkinson’s Disease)
Intravenous glutathione has been explored as an adjunct in Parkinson’s disease in several small clinical and observational studies.
A commonly cited randomized, double-blind, placebo-controlled pilot trial evaluated IV glutathione 1,400 mg administered three times weekly for 4 weeks in patients with idiopathic Parkinson’s disease whose symptoms were not fully controlled by standard therapy.7
The primary objectives of this study were to assess feasibility, safety, and tolerability, with exploratory analyses of motor outcomes.
Earlier open-label evaluations used IV glutathione doses ranging from approximately 600 to 1,400 mg several times per week.8-10
However, these studies were generally small, of short duration, and not powered to draw definitive conclusions. Current literature views IV glutathione in Parkinson’s disease as investigational rather than established therapy.
5.2 Peripheral Obstructive Arterial Disease
A randomized, double-blind, placebo-controlled trial evaluated intravenous glutathione administered twice daily for 5 days in patients with peripheral obstructive arterial disease.11
In this study, the glutathione group demonstrated statistically significant changes in pain-free walking distance and selected macro- and microcirculatory parameters compared with placebo.
Because the trial included a limited number of participants and was of short duration, the findings are considered preliminary and exploratory.
These results have been interpreted as hypothesis-generating with respect to redox modulation in peripheral arterial disease, but they have not led to broader clinical adoption.
Intravenous glutathione remains an investigational adjunct in this setting, and additional well-designed studies would be needed to clarify any clinical relevance.
5.3 Respiratory and Infectious Conditions
Glutathione plays an important role in pulmonary antioxidant defenses, and altered glutathione balance has been described in chronic obstructive pulmonary disease (COPD), asthma, and other inflammatory lung disorders. 1-2,12
These mechanistic observations have contributed to scientific interest in whether exogenous glutathione might influence redox biology in respiratory settings, although they do not establish a therapeutic role for intravenous glutathione.
During the COVID-19 pandemic, intravenous glutathione was evaluated in limited human studies. A small randomized, controlled investigation assessed the feasibility and safety of IV glutathione administered as an adjunct to standard care in patients with moderate COVID-19 and respiratory compromise.14
In this exploratory trial, differences in certain clinical or laboratory parameters were reported between treatment groups, but the study was not powered to determine efficacy, and the findings should be interpreted cautiously. Case reports have also described the investigational use of glutathione in COVID-19–related respiratory illness,13 but such uncontrolled observations cannot establish safety or effectiveness, and additional research is needed.
5.4 Metabolic and Endocrine Conditions
Oxidative stress has been implicated in the pathogenesis of insulin resistance and type 2 diabetes mellitus.1
Published investigations of glutathione in metabolic disorders have primarily evaluated oral supplementation or glutathione precursors, rather than intravenous administration. 15
These studies have reported changes in selected oxidative stress markers, but their findings are limited to non-IV formulations and do not establish any therapeutic role for intravenous glutathione in metabolic or endocrine conditions.
In the reproductive literature, oxidative stress has also been discussed as a factor affecting sperm function and male infertility. 16-17
Reviews in this area describe antioxidant mechanisms, including those involving glutathione, as part of broader redox biology. However, these discussions are largely mechanistic, and no clinical studies have evaluated intravenous glutathione for infertility or reproductive disorders.
5.5 Dermatologic and Aesthetic Uses (Skin Lightening)
Glutathione has been discussed in dermatology literature because of its biochemical involvement in melanogenesis pathways and antioxidant activity. Most published evaluations of glutathione for cosmetic skin-lightening purposes involve oral or topical formulations, and the clinical evidence base remains limited.18
Intravenous glutathione has been promoted in some regions for aesthetic use; however, such use lacks support from controlled clinical studies, and has been the subject of advisories from several regulatory and professional bodies citing insufficient evidence of safety and effectiveness. At present, no well-designed clinical trials have established the safety or efficacy of IV glutathione for skin lightening, and its cosmetic use is considered investigational.
5.6 Autism Spectrum Disorder (ASD)
Glutathione plays an important role in cellular redox regulation, mitochondrial function, and antioxidant defense. Abnormalities in glutathione metabolism have been reported in individuals with autism spectrum disorder (ASD), including reduced glutathione levels, increased oxidative stress markers, and alterations in redox-related biochemical pathways.19
These observations have prompted scientific interest in glutathione-related mechanisms in ASD.
At the time of this writing, no published controlled clinical trials have evaluated intravenous glutathione as a therapeutic intervention for ASD, and available data do not define its safety or efficacy in this population.
6. SAFETY PROFILE AND ADVERSE EFFECTS
Across published IV glutathione studies in adults, treatment has generally been described as well tolerated, with most reported adverse events typically mild, transient, and nonspecific.7-8,14 Observed reactions include:
Local / Infusion-Site Reactions
-
Erythema or irritation at the infusion site7
-
Localized pain or induration8
-
Thrombophlebitis 8
Neurologic
-
Dizziness or lightheadedness7
-
Headache7
Gastrointestinal
Dermatologic
In the small clinical studies conducted to date, no clear drug-related serious adverse reactions have been identified. However, these studies were limited in sample size and duration, and the available data are insufficient to define the safety profile of repeated or long-term intravenous administration.
Regulatory agencies have also documented clusters of adverse events, such as fever and chills, associated with injectable glutathione products that were later found to contain high endotoxin levels.20
These incidents reflect the consequences of contamination or inadequate manufacturing controls rather than inherent properties of glutathione itself, underscoring the importance of using products prepared with appropriate raw-material sourcing, validated sterile compounding processes, and rigorous endotoxin and sterility testing.
Any intravenous preparation should be obtained from facilities operating under suitable quality and compliance standards (e.g., current good manufacturing practice), with appropriate monitoring for adverse reactions during administration.
7. FORMULATION, STABILITY, AND HANDLING (SCIENTIFIC OVERVIEW)
Reduced glutathione in aqueous solution is sensitive to oxidative and temperature-dependent degradation. Because intravenous preparations must also meet stringent sterility and endotoxin requirements, these properties have several implications for formulation and handling:
-
Oxidation: Dissolved GSH can oxidize to GSSG and other degradation products when exposed to oxygen or trace catalytic impurities. Formulation approaches may therefore include strategies to limit oxygen exposure during preparation and storage.
-
Temperature: Elevated temperatures accelerate both oxidative and hydrolytic degradation processes. As a result, glutathione-containing injectable solutions are often stored under refrigerated conditions, as supported by product-specific stability data, to help maintain potency and quality throughout their assigned shelf life.
-
Sterility and endotoxin control: Glutathione solutions are typically sterilized by filtration rather than terminal heat sterilization due to the compound’s thermal sensitivity. Consequently, validated aseptic processing, container–closure integrity, and stringent microbial and endotoxin controls are essential considerations for producing a parenteral-grade preparation.
8. REGULATORY AND QUALITY CONSIDERATIONS (HIGH LEVEL SUMMARY)
Regulatory agencies in several jurisdictions have issued communications regarding the compounding and use of glutathione for injection. Common themes in these statements include:
-
Raw material quality: Concerns about the use of dietary-grade or non-pharmaceutical-grade glutathione as a starting material for sterile injectable preparations, due to risks of microbial contamination and elevated endotoxin levels.
-
Compliance with sterile manufacturing standards: Emphasis on adherence to applicable Good Manufacturing Practice (GMP) requirements when producing sterile glutathione formulations. These expectations include validated sterilization or sterile filtration processes, appropriate environmental monitoring, and robust quality control testing.
Healthcare practitioners should be aware of federal, state, and local regulations governing the sourcing, compounding, and distribution of intravenous glutathione, including any requirements specific to 503B outsourcing facilities and compounding pharmacies. Intravenous preparations should be used only if they are manufactured under appropriate sterile-processing and quality-assurance standards.
9. SUMMARY
Reduced L-glutathione is a central component of human antioxidant defense and cellular redox regulation. Intravenous formulations have been explored in a variety of clinical and investigational contexts where oxidative stress and inflammation are thought to contribute to disease processes.
Pharmacokinetic evaluations show that IV glutathione produces a rapid but transient increase in circulating levels, followed by quick clearance through oxidation and metabolism. Clinical research across neurodegenerative, vascular, respiratory, metabolic, and dermatologic settings has yielded heterogeneous and generally preliminary findings, with most studies limited by small sample sizes, short durations, and variability in design.
Short-term controlled studies have described IV glutathione as generally well tolerated, but the available evidence base remains limited, and longer-term safety has not been defined. Existing reports underscore the importance of using appropriately manufactured sterile preparations and maintaining routine monitoring during administration.
Further research is needed to clarify the pharmacologic behavior, safety characteristics, and scientific relevance of intravenous glutathione in different investigational settings.
10. SELECTED REFERENCES
-
Ballatori N, et al. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390(3):191-214.
-
Espinosa-Díaz C, et al. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183-197.
-
Lu S. Regulation of Glutathione Synthesis. Mol Aspects Med. 2009;30(1-2):42-59.
-
Witschi A, et al. The systemic availability of oral glutathione. Eur J Clin Pharmacol. 1992;43:667-669.
-
Vaskova J, et al. Glutathione-Related Enzymes and Proteins: A Review. Molecules. 2023 Feb 2;28(3):1447.
-
Hong SY, et al. Pharmacokinetics of glutathione and its metabolites in normal subjects. J Korean Med Sci. 2005 Oct;20(5):721-6.
-
Hauser RA, et al. Randomized, double-blind, pilot evaluation of intravenous glutathione in Parkinson’s disease. Mov Disord. 2009;24:979-983.
-
Sechi G, et al. Reduced intravenous glutathione in the treatment of early Parkinson’s disease. Prog Neuro-Psychopharmacol & Biol Psychiat. 1996;20:1159-70.
-
Wang H, et al. Potential use of glutathione as a treatment for Parkinson’s disease. Exp Ther Med. 2021 Feb;21(2):125. doi: 10.3892/etm.2020.9557.
-
Otto M. et al. The use of intravenous glutathione for symptom management of Parkinson’s disease: A case report. Altern Ther Health Med. Jul 2018;24(4):56-60.
-
Arosio E, et al. Effect of glutathione infusion on leg arterial circulation, cutaneous microcirculation, and pain-free walking distance in patients with peripheral obstructive arterial disease. Mayo Clin Proc. 2002;77:754-759.
-
Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16(3):534-554.
-
Horowitz R., Freeman P., Bruzzese J. Respiratory Med Case Reports. 2020:30:101063. doi: 10.1016/j.rmcr.2020.101063.
-
Dewan B, Shinde S. Glutathione as an adjuvant therapy for acute respiratory distress syndrome associated with COVID-19 infection. J Adv Med Med Res. 2022;34(22):100-113.
-
Kalamkar S. et al. Randomized clinical trial of how long-term glutathione supplementation offers protection from oxidative damage and improves HbA1c in elderly Type 2 diabetic patients. Antioxidants. 2022;11,1026.
-
Adeoye O. et al. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist Repro. 2018;22(1):61-66.
-
Majzoub A.; Agarwal A. Antioxidant therapy in idiopathic oligoasthenoteratozoospermia. Ind J Urol. Jul/Sept 2017;33(3):207-214.
-
Davids LM, et al. Intravenous glutathione for skin lightening: a review of the evidence and safety concerns. S Afr Med J. 2016;106(8):782-786.
-
Kern J, et al. Evidence of neurodegeneration in autism spectrum disorder. Transl Neurodegener. 2013 Aug 8;2:17. doi: 10.1186/2047-9158-2-17.
-
U.S. Food and Drug Administration. FDA highlights concerns with using dietary ingredient glutathione to compound sterile injectables. Safety communication, 2019.
Copyright © 2025 McGuff Outsourcing Solutions. All rights reserved.
No part of this document may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or electronic transmission, without the prior written permission of McGuff Outsourcing Solutions.